Wikijunior:The Elements/Scandium
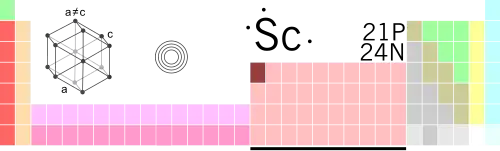


What does it look, feel, or taste like?
Scandium is a soft metal with a silvery appearance. It develops a slightly yellowish or pinkish cast when oxidized by air. It has no distinctive odor, nor any recognizable taste.
How was it discovered?
The element scandium was discovered because an element that was previously unknown was shown in Mendeleev's periodic table. Mendeleev had seen a gap between calcium and titanium.
Lars Fredrik Nilson specifically looked for the unknown element and found it in 1879. Scandium’s atomic number fit into the gap as predicted.
Where did its name come from?
Lars Frederik Nilson named the new element scandium because it had been discovered in Sweden. Scandia is the Latin word for Scandinavia.
Where is it found?
Scandium is found in larger deposits in only a few minerals, such as thortveitite and bazzite. Thortveitite is primarily found in Norway. Smaller occurrences have been reported in Japan and Russia. In the United States, it is found in North Carolina and Colorado. Bazzite has been identified in Italy, France, and Switzerland.
Scandium does not occur freely in nature, but trace amounts are present in over 800 known minerals. It ranks as the 50th most abundant element in Earth’s crust.
Scandium has also been detected in the atmosphere of the Sun!
What are its uses?
Scandium helps make very bright lights. It is used in mercury vapor lamps that light up places like stadiums and sports arenas. It makes the light brighter and more natural like sunshine. People can see night games clearly.
When scandium is mixed with other metals, it creates strong materials called alloys. Aluminum-scandium alloys increase strength and are recyclable. They are used in bicycles, baseball bats, airplane parts, and electronics Aluminum-scandium alloys are used in 3D printing, to create tiny parts for tools and machines.
Titanium-scandium alloys are very light weight and help prevent corrosion. These are used in prosthetics, aerospace, and advanced weaponry.
Magnesium-scandium alloys reduce weight in racing bikes.
Is it dangerous?
Scandium is dangerous in several ways. Scandium powder burns easily. It can ignite quickly when exposed to air or heat. In addition, long-term exposure to its vapors may harm lungs and cause respiratory problems. Ingesting small amounts over a long period may cause liver damage. People who work in labs, mining, or manufacturing may be at higher risk. They need to follow safety procedures and wear protective clothing.
References
Chemistry Learner. (2025). Scandium: What is scandium? Chemistry Learner. https://www.chemistrylearner.com/scandium.html
Kiddle Encyclopedia. (2025, July 11). Scandium facts for kids. https://kids.kiddle.co/Scandium
Kiddle Encyclopedia. (2025, June 12). Mercury-vapor lamp facts for kids. Kiddle. https://kids.kiddle.co/Mercury-vapor_lamp
KidsKonnect. (2025, September 4). Scandium facts & worksheets. KidsKonnect. https://kidskonnect.com/science/scandium
Stanford Advanced Materials. (2025). 3 types of scandium alloys: A simple introduction. Scandium.org. https://www.scandium.org/3-types-of-scandium-alloys-a-simple-introduction/
-
 flammable
flammable